Unraveling the NaHS Mystery: Why Does Sodium Hydrosulfide Smell Like "Rotten Eggs"?
Sodium Hydrosulfide (NaHS) is a critical compound used extensively across industries, from leather processing and mineral flotation to the synthesis of dyes and pharmaceuticals. Yet, many professionals encountering NaHS—especially its solutions—are instantly met with a distinctive, unpleasant odor: the classic "rotten egg smell." What is the chemical basis for this characteristic scent? Today, we delve into the chemistry behind the odor to provide clarity and crucial safety insights.
The Culprit: Hydrogen Sulfide (H2S)
To understand the "rotten egg smell" associated with NaHS, we must first identify the primary compound responsible: Hydrogen Sulfide (H2S).
Hydrogen sulfide is a colorless, highly toxic gas renowned for its repulsive odor. It occurs naturally in volcanic gases and is a common byproduct of the anaerobic decomposition of organic matter.
The Chemical Connection: How H2S is Released from NaHS
While pure, dry Sodium Hydrosulfide (NaHS) is theoretically odorless, it is a highly reactive chemical. The odor arises when NaHS comes into contact with moisture, air, or acidic substances.
The release of H2S is primarily driven by the following chemical reactions:
Hydrolysis in Water (The Main Source):
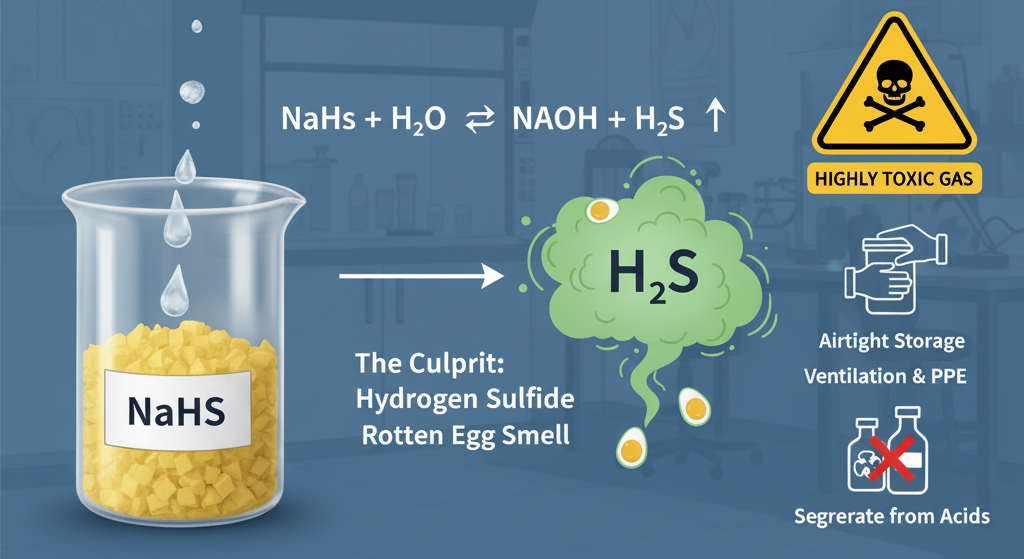
When NaHS dissolves in water (or absorbs moisture from the air), it undergoes hydrolysis, releasing trace amounts of hydrogen sulfide gas:
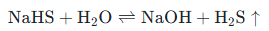
(Sodium Hydrosulfide + Water ⇌ Sodium Hydroxide + Hydrogen Sulfide)
Reaction with Atmospheric Carbon Dioxide (CO2):
Carbon dioxide in the air is slightly acidic. It reacts with NaHS to release H2S:
(Sodium Hydrosulfide + Carbon Dioxide + Water → Sodium Bicarbonate + Hydrogen Sulfide)
Reaction with Acids:
In the presence of stronger acids, the reaction accelerates, leading to a rapid and dangerous release of large volumes of H2S.
It is the constant, low-level generation of H2S from these reactions that produces the characteristic "rotten egg smell." This odor serves as an essential warning signal, as H2S is an industrial hazard.
Safety Protocol and Storage Recommendations
Hydrogen sulfide is highly toxic. Crucially, high concentrations can rapidly fatigue the olfactory nerves, causing a loss of smell—a dangerous condition known as olfactory paralysis. Therefore, strict safety protocols are non-negotiable when handling NaHS:
- Airtight Storage: Ensure all NaHS products (solid flakes or solution) are stored in tightly sealed containers, minimizing exposure to air and moisture.
- Ventilation: Operations involving NaHS must always be conducted in well-ventilated areas or under local exhaust ventilation (LEV). Appropriate Personal Protective Equipment (PPE), including H2S monitoring systems and respirators, should be mandatory.
- Segregation: Strictly separate NaHS from all acidic materials to prevent rapid, exothermic H2S release.
- Emergency Planning: Establish and regularly drill emergency procedures for potential $\text{H}_2\text{S}$ leaks.
Baijin Chemical's Commitment to Safety
As a dedicated supplier of Sodium Hydrosulfide, Baijin Chemical prioritizes product stability and safety. Leveraging our 30 years of expertise in hazardous chemical management, we employ stringent controls throughout our production, packaging, and transportation processes to ensure product purity and minimize the risk of premature H2S generation.
By understanding the chemical origin of the odor, we aim to empower our clients to handle this vital chemical more safely and professionally. For detailed safety data sheets or professional consultation on NaHS storage and usage, please contact our expert team.